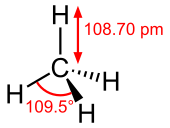The neutral density (  ) or empirical neutral density is a density variable used in oceanography, introduced in 1997 by David R. Jackett and Trevor McDougall.[1] It is a function of the three state variables (salinity, temperature, and pressure) and the geographical location (longitude and latitude). It has the typical units of density (M/V). Isosurfaces of
) or empirical neutral density is a density variable used in oceanography, introduced in 1997 by David R. Jackett and Trevor McDougall.[1] It is a function of the three state variables (salinity, temperature, and pressure) and the geographical location (longitude and latitude). It has the typical units of density (M/V). Isosurfaces of  form “neutral density surfaces”, which are closely aligned with the "neutral tangent plane". It is widely believed, although this has yet to be rigorously proven, that the flow in the deep ocean is almost entirely aligned with the neutral tangent plane, and strong lateral mixing occurs along this plane ("epineutral mixing") vs weak mixing across this plane ("dianeutral mixing"). These surfaces are widely used in water mass analyses. Neutral density is a density variable that depends on the particular state of the ocean, and hence is also a function of time, though this is often ignored. In practice, its construction from a given hydrographic dataset is achieved by means of a computational code (available for Matlab and Fortran), that contains the computational algorithm developed by Jackett and McDougall. Use of this code is currently restricted to the present day ocean.
form “neutral density surfaces”, which are closely aligned with the "neutral tangent plane". It is widely believed, although this has yet to be rigorously proven, that the flow in the deep ocean is almost entirely aligned with the neutral tangent plane, and strong lateral mixing occurs along this plane ("epineutral mixing") vs weak mixing across this plane ("dianeutral mixing"). These surfaces are widely used in water mass analyses. Neutral density is a density variable that depends on the particular state of the ocean, and hence is also a function of time, though this is often ignored. In practice, its construction from a given hydrographic dataset is achieved by means of a computational code (available for Matlab and Fortran), that contains the computational algorithm developed by Jackett and McDougall. Use of this code is currently restricted to the present day ocean.
Dianeutral mixing in the thermocline of the Indian Ocean
Abstract
The water mass structure in the main thermocline of the Indian Ocean suggests that the thermocline water of the northern Indian Ocean must be replenished from the southern hemisphere. The transformation of the southern Indian Ocean water could be either by epineutral advection (advection along neutral surfaces), epineutral diffusion (diffusion along neutral surfaces), dianeutral advection (advection across neutral surfaces) or dianeutral diffusion (diffusion across neutral surfaces). This paper points to the importance of dianeutral mixing processes in achieving this water-mass transformation.
Five neutral surfaces, which span the main thermocline of the Indian Ocean from about 150 to 750 m in the northern Indian Ocean, are mapped to examine the dianeutral mixing processes in the thermocline. Dianeutral mixing and advection processes, including the two advection processes, cabbeling and thermobaricity, are evaluated and plotted on those neutral surfaces and in profiles at several places. It is found that in the northern Indian Ocean dianeutral turbulent mixing leads to mainly upward dianeutral advection, while in the southern Indian Ocean the same process causes mainly downward dianeutral advection. Cabbeling and thermobaricity together produce a maximum dianeutral velocity of -0.2x 10−7 m s−1 off northwest Australia (south of the Australasian Mediterranean Water) in the upper thermocline, while in the lower thermocline this maximum dianeutral velocity is found in the northwest Indian Ocean and north of the Australasian Mediterranean Water. The strongest dianeutral velocity caused by the vertical turbulent mixing is found in the upper thermocline off northeast Africa with a value of 2 x 10−7 m s−1 r when a vertical diffusivity D of 10−5 m2 s−1 is assumed. The most striking features are found in two meridional sections, one in the western Indian Ocean at 60°E and another in the eastern Indian Ocean at 90°E, which show that the Indian Central Water formation in the southern hemisphere is characterized by a tongue-like pattern of downward dianeutral advection extending from its formation region (about 40°–45°S) towards the equator in a depth range between about 300 and 800 m, centred at 600–700 m. Moreover, the tongue-like pattern corresponds to another tongue-like pattern of large positive Turner angle centred at 400 m, 200 m above the former, which means that the northward extending Central Water is characterized by strong fingering and suggests that the former tongue is also associated with the double-diffusive flux of salinity. This is a typical example of Central Water formation proposed by Iselin (1939) and Stommel (1979), that the water in the main thermocline is formed at the surface in late winter and subsequently moves towards the equator along isopycnal surfaces. It is also consistent with the transition path of Indian Central Water from the southern hemisphere to the northern Indian Ocean proposed by You and Tomczak (1993), wherein Indian Central Water is subducted in latitudes 40°–45°S, is advected with the subtropical gyre, and finds its way into the northern Indian Ocean through western boundary currents along the σθ= 26.7 isopycnal surface.
Methane
From Wikipedia, the free encyclopedia
Jump to navigationJump to searchFor the emergency service protocol, see
ETHANE.
Methane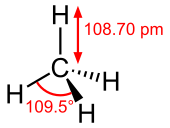 |
|
| Names |
|---|
| Preferred IUPAC name |
Systematic IUPAC nameCarbane (never recommended [1]) |
Other names- Marsh gas
- Natural gas
- Carbon tetrahydride
- Hydrogen carbide
|
| Identifiers |
|---|
| |
| |
| 3DMet | |
| 1718732 |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.000.739  |
| EC Number | |
| 59 |
| KEGG | |
| MeSH | Methane |
| |
| RTECS number | |
| UNII | |
| UN number | 1971 |
| |
|
|
| Properties |
|---|
| CH4 |
| Molar mass | 16.043 g·mol−1 |
| Appearance | Colorless gas |
| Odor | Odorless |
| Density | - 0.657 kg·m−3 (gas, 25 °C, 1 atm)
- 0.717 kg·m−3 (gas, 0 °C, 1 atm)[2]
- 422.8 g·L−1 (liquid, −162 °C)[3]
|
| Melting point | −182.456 °C (−296.421 °F; 90.694 K)[3] |
| Boiling point | −161.5 °C (−258.7 °F; 111.6 K)[3] |
| Critical point (T, P) | 190.56 K (−82.59 °C; −116.66 °F), 4.5992 megapascals (45.391 atm) |
| 22.7 mg·L−1[4] |
| Solubility | Soluble in ethanol, diethyl ether, benzene, toluene, methanol, acetone and insoluble in water |
| log P | 1.09 |
| 14 nmol·Pa−1·kg−1 |
| Conjugate acid | Methanium |
| Conjugate base | Methyl anion |
| −17.4×10−6 cm3·mol−1[5] |
| Structure |
|---|
| Td |
| Tetrahedron |
| 0 D |
| Thermochemistry[6] |
|---|
| 35.7 J·(K·mol)−1 |
| 186.3 J·(K·mol)−1 |
| −74.6 kJ·mol−1 |
| −50.5 kJ·mol−1 |
| −891 kJ·mol−1 |
| Hazards[7] |
|---|
| GHS labelling: |
|  |
| Danger |
| H220 |
| P210 |
| NFPA 704 (fire diamond) | |
| Flash point | −188 °C (−306.4 °F; 85.1 K) |
| 537 °C (999 °F; 810 K) |
| Explosive limits | 4.4–17% |
| Related compounds |
|---|
Related alkanes | |
| Supplementary data page |
|---|
| Methane (data page) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). |
Methane ( MEH-thayn, MEE-thayn) is a chemical compound with the chemical formula CH4 (one carbon atom bonded to four hydrogen atoms). It is a group-14 hydride, the simplest alkane, and the main constituent of natural gas. The relative abundance of methane on Earth makes it an economically attractive fuel, although capturing and storing it poses technical challenges due to its gaseous state under normal conditions for temperature and pressure.
Naturally occurring methane is found both below ground and under the seafloor and is formed by both geological and biological processes. The largest reservoir of methane is under the seafloor in the form of methane clathrates. When methane reaches the surface and the atmosphere, it is known as atmospheric methane.[9] The Earth's atmospheric methane concentration has increased by about 150% since 1750, and it accounts for 20% of the total radiative forcing from all of the long-lived and globally mixed greenhouse gases.[10] It has also been detected on other planets, including Mars, which has implications for astrobiology research.[11]
A very approximate estimate of the area-integrated total mean dianeutral fluxes north of 32°S shows net dianeutral upwelling transport of about 1.4 Sv (1 Sv= 106 m3 s−1) across the σθ=27.3 neutral surface in the lower thermocline and about 4.6 Sv across the σθ=25.7 neutral surface in the upper thermocline. These are very significant net upward dianeutral volume transports, about one nineteenth and one sixth, respectively, of the 27 Sv geopotential upwelling transport estimated by Toole and Warren (1993) at 2000 m depth. About three times more upward transport across the uppermost neutral surface than across the lowermost neutral surface suggests, that for the dianeutral flux alone, there is a hint of the need for a supply of water from the southern Indian Ocean between the upper and lower thermocline. Results contained in this paper clearly indicate that dianeutral mixing processes play a vital role in achieving water mass conversion and thermocline ventilation in the northern Indian Ocean. however, one should be aware that some of the results calculated here have included the assumed constant epineutral diffusivity K of 103m2s−1 and dianeutral diffusivity D of 10−5m2s−1